How Isotopes Help Us Understand Earth's Past
Your mother and I have been meaning to tell you this for a while now... isotopes have nothing to do with ice. No, really, it's true. Regardless, isotopes are pretty awesome, and some really smart people are using them to understand what Earth was like back in the good ol' days - like, millions of years ago! One of the main reasons why it's important to understand Earth's history, is so that we can have a better understanding of its future. Earth is no stranger to climate change - believe me, the pair have history - and if we can understand how the Earth responded to major climatic changes in the past, we can predict how it will respond in the future. Then we can prepare for the Anthropogenic apocalypse...
 |
| Personally, I'm hoping for a Mad Max apocalypse, or really, anything but the apocalypse from the Road... oh god, anything but the Road |
So, what are isotopes, and how do they help us understand the past? Elements such as Carbon and Oxygen have a certain number of protons and neutrons, and the most common versions of these elements tend to have an equal amount of protons and neutrons. For example, Oxygen 16 gets its name from the fact that it has 8 protons and 8 neutrons (8+8=16, in case you were wondering). However, some versions of Oxygen have 8 protons and 9 neutrons, or 10 neutrons. The number of protons never change - this is what identifies an element as Oxygen, or Carbon, or any other element - but the number of neutrons can. These variations are known as isotopes, and they can be stable or unstable (radioactive). Both stable and unstable isotopes can be found in what are known as Natural Archives - trees, seas, ice caps (okay, isotopes do have something to do with ice), fossils, sediments and algae. Measuring changes in the concentration of these isotopes, can give us an idea of what the world was like when these isotopes were archived.
 |
| Even the trees have stories to tell... and their grandchildren have heard them way too many times |
Unsurprisingly, the method by which isotopes are used to reconstruct Earth's past climate is complex and deeply nuanced. Consider the example of algae containing oxygen isotopes. The purity of the algae sample is paramount, because even the slightest contamination will heavily influence the isotopic value, which will in turn affect our understanding of its composition, and its implications for our understanding of the past climate. As a result, the algae sample will have to be scrutinised in painstaking detail, with even the nuances of the environment from which it was collected being examined to see how the environment might have affected the isotopic value. Contamination is combatted through various methods ranging from inducing chemical reactions to sieving. The exact method will vary based off the nature and magnitude of contamination. Now that that's out the way, we can begin actually extracting the oxygen isotope. Well... not exactly. You can't just extract the oxygen isotope, you first have to remove the hydrous outer layer of the algae by applying heat or oxidising reagents - which sound cooler than they are - and then separate the oxygen from the silica skeleton. You won't be surprised to hear that the skeleton itself is complex in its layering and chemistry, and there are very many factors to consider when inducing chemical reactions to separate the oxygen and the skeleton.
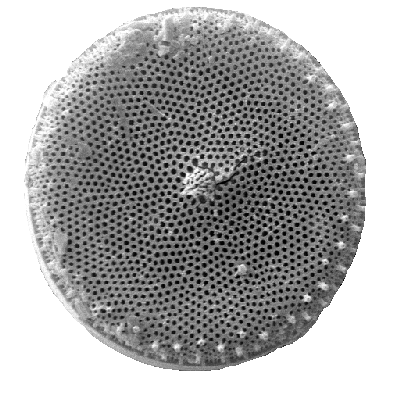 |
| If you look at the skeleton long enough, it'll start moving! |
Comments
Post a Comment